Sickle cell disease is a debilitating and painful inherited blood disorder that primarily affects Black people in the United States.
Currently, the only cure for this condition is a bone marrow transplant, which is a difficult and risky procedure.
However, there is hope that a new cure may soon be available that attacks the disorder at its genetic source.
On Tuesday, advisers to the Food and Drug Administration will review a gene therapy for sickle cell disease that uses CRISPR, the groundbreaking gene editing tool that won its inventors the Nobel Prize in 2020.
If approved by the FDA, this would be the first gene therapy on the U.S. market based on CRISPR. However, the advisers will also consider whether more research is needed to fully understand the potential unintended consequences of the treatment.
The development of a gene therapy for sickle cell disease could be a major breakthrough in the treatment of this condition, providing a less invasive and more effective option for patients.
In early December, the agency is expected to make a decision regarding the treatment for sickle cell disease, followed by the consideration of a different gene therapy for the same condition later that month. Dr. Allison King, a professor at Washington University School of Medicine in St. Louis, who specializes in caring for children and young adults with sickle cell disease, expresses her enthusiasm towards the potential of new treatments.
She believes that any intervention that can alleviate the pain and numerous health complications associated with this condition is truly remarkable.
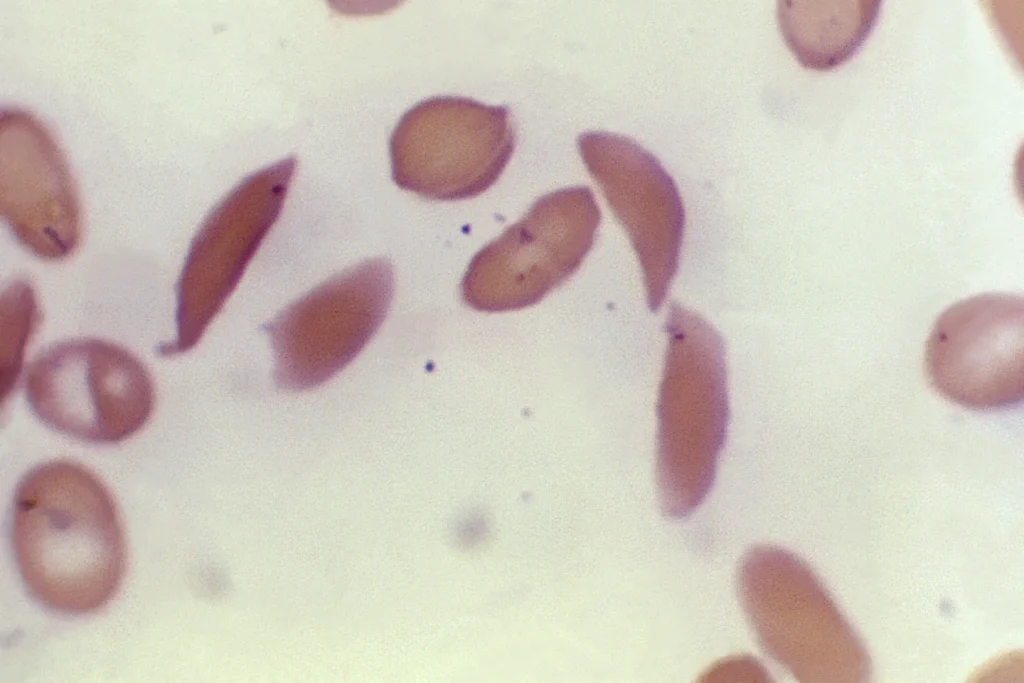
Sickle cell disease is characterized by a genetic mutation that affects hemoglobin, the protein responsible for carrying oxygen in red blood cells.
This mutation causes the cells to take on a crescent shape, leading to the blockage of blood flow and resulting in excruciating pain, organ damage, stroke, and other serious issues.
The disease affects millions of individuals worldwide, with approximately 100,000 cases in the United States alone.
It is more prevalent in regions where malaria is or was common, such as Africa and India, and is also more common among certain ethnic groups, including individuals of African, Middle Eastern, and Indian descent. Scientists believe that carrying the sickle cell trait provides some protection against severe malaria.
Sickle cell disease is a genetic disorder that affects millions of people worldwide. The disease causes red blood cells to become misshapen and break down, leading to a range of complications such as chronic pain, organ damage, and increased risk of infections.
Current treatments for sickle cell disease include medications and blood transfusions, which can alleviate symptoms but do not offer a permanent cure.
The only permanent solution is a bone marrow transplant, which is a complex and risky procedure that requires a closely matched donor without the disease.
However, a new treatment called “exa-cel,” developed by Vertex Pharmaceuticals and CRISPR Therapeutics, offers a promising alternative.
This one-time gene therapy involves permanently changing the DNA in a patient’s blood cells, allowing the body to produce a fetal form of hemoglobin that is naturally present at birth but then switches to an adult form that’s defective in people with sickle cell disease.
The treatment involves removing stem cells from the patient’s blood and using CRISPR to knock out the switching gene.
After killing off other flawed blood-producing cells, the altered stem cells are then given back to the patient.
While the treatment has been tested in a relatively small number of patients, it represents a significant step forward in the search for a cure for sickle cell disease.
In a briefing document released on Friday prior to the advisory committee meeting, Vertex Pharmaceuticals provided an update on their groundbreaking treatment.
The document revealed that a total of 46 individuals participated in the pivotal study, with 30 of them having a minimum of 18 months of follow-up.
Astonishingly, 29 out of these 30 patients remained free of pain crises for at least a year, and all 30 managed to avoid hospitalization due to pain crises for the same duration.
Vertex Pharmaceuticals hailed this treatment as “transformative” and emphasized its strong safety profile.
During a scientific conference earlier this year, Victoria Gray, the first patient to undergo this treatment, shared her remarkable experience with researchers.
Gray had endured excruciating bouts of pain since childhood and had relied on high-dose pain medications and occasional blood transfusions.
However, she described feeling an extraordinary sense of rebirth on the day she received the gene therapy.
Thanks to the treatment, Gray is now able to engage in activities such as running around with her children and maintaining a full-time job. She expressed immense relief that her children no longer fear losing their mother to sickle cell disease.
Despite the overwhelmingly positive results, the FDA has requested the input of an external panel of gene therapy experts in an upcoming meeting to address a lingering concern associated with CRISPR technology.
This concern revolves around the potential for “off-target effects,” which refer to unexpected and undesired alterations in an individual’s genome.
The FDA seeks guidance on whether Vertex Pharmaceuticals’ research on these effects was sufficiently comprehensive to assess the associated risks or if further studies are necessary.
While the FDA is not obligated to follow the panel’s advice, it often takes their recommendations into serious consideration.
The topic of gene therapy for sickle cell disease has garnered significant attention in recent years, as it holds the potential to revolutionize treatment options for patients suffering from this debilitating condition.
In this regard, the approval of a second gene therapy by the FDA, developed by Bluebird Bio, is eagerly anticipated, and its impact on the market and patient outcomes is eagerly awaited.
Bluebird Bio’s gene therapy differs from existing treatments in its approach. Instead of merely managing symptoms, this therapy seeks to address the root cause of sickle cell disease by introducing functional copies of a modified gene.
These copies enable red blood cells to produce “anti-sickling” hemoglobin, which can prevent or reverse the formation of misshapen cells.
This innovative approach holds great promise, as it has the potential to provide long-lasting relief and potentially even a cure for individuals suffering from sickle cell disease.
However, before the therapy can be made available on the market, it is essential to ensure its safety and efficacy. To address this concern, Bluebird Bio has proposed a post-approval safety study.
This study will allow for the collection of data on the therapy’s long-term effects and any potential risks associated with its use.
By conducting such a study, the company demonstrates its commitment to ensuring patient safety and the continued improvement of the therapy.
In addition to the post-approval safety study, product labeling that outlines potential risks is also proposed.
This labeling will provide healthcare professionals and patients with essential information regarding the therapy, including any known side effects or contraindications.
By clearly communicating this information, the company aims to empower patients and healthcare providers to make informed decisions about the use of the therapy.
Furthermore, the institute report mentioned in the topic suggests that prices up to approximately $2 million for these gene therapies would be considered cost-effective.
While the companies involved have not released specific pricing information, this figure provides some insight into the potential cost of these groundbreaking treatments.
It is important to note that this cost is being compared to the significant medical expenses associated with current sickle cell treatments.
Research conducted earlier this year indicates that medical expenses for existing treatments for sickle cell disease can amount to approximately $1.6 million for women and $1.7 million for men over their lifetime.
Therefore, if the gene therapies can provide long-term benefits and potentially even a cure, the higher upfront cost may be justified by the potential savings in long-term medical expenses.
In conclusion, the pending FDA decision on Bluebird Bio’s gene therapy for sickle cell disease marks a significant milestone in the field of genetic medicine.
If approved, this therapy has the potential to revolutionize treatment options for patients suffering from this debilitating condition.
The proposed post-approval safety study, product labeling outlining potential risks, and continuing research efforts demonstrate the company’s commitment to ensuring patient safety and the continued improvement of the therapy.
While pricing information is yet to be released, the institute report suggests that prices up to approximately $2 million would be considered cost-effective, considering the substantial medical expenses associated with current sickle cell treatments.
Overall, the approval of this gene therapy holds great promise for patients and represents a significant advancement in the fight against sickle cell disease.
Dr. King, a highly esteemed medical professional based in St. Louis, astutely recognized the potential financial burden associated with the implementation of these novel treatments.
However, she posed a thought-provoking question that compelled one to ponder the true value of alleviating suffering and enhancing the quality of life for individuals afflicted by debilitating conditions.
Indeed, the cost may be significant, but when contemplating the immeasurable benefits of enabling patients to experience a life free from agony and the constant confinement of hospital walls, one cannot help but question whether any price is too high to pay.
The pursuit of improved healthcare outcomes necessitates a willingness to invest in groundbreaking therapies that have the potential to revolutionize the way we approach patient care, ultimately allowing individuals to lead fulfilling and pain-free lives.
