The recent authorization of the world’s first gene therapy treatment for sickle cell disease by Britain’s medicines regulator marks a significant milestone in the field of medical science.
This groundbreaking development has the potential to bring relief to thousands of individuals suffering from this debilitating disease in the United Kingdom.
The approval of Casgevy, a medicine utilizing the gene editing tool CRISPR, represents a major advancement in the treatment of sickle cell disease and thalassemia.
The Medicines and Healthcare Regulatory Agency’s decision to approve Casgevy is a testament to the immense potential of gene therapy in revolutionizing the treatment of genetic disorders.
This milestone is particularly notable as it represents the first time that a medicine utilizing CRISPR has been licensed for use, an achievement that was recognized with a Nobel Prize in 2020.
The approval of Casgevy signifies a new era in the treatment of sickle cell disease, offering hope to patients who have long faced limited options for long-lasting treatment.
Casgevy, developed by Vertex Pharmaceuticals (Europe) Ltd. and CRISPR Therapeutics, has been authorized for use in patients with sickle cell disease and thalassemia who are 12 years old and over.
This approval opens up new possibilities for individuals with these conditions, providing them with access to a treatment that has the potential to significantly improve their quality of life.
In the past, the only long-lasting treatment for sickle cell disease has been bone marrow transplants, which are extremely arduous procedures associated with unpleasant side effects.
The approval of Casgevy represents a major step forward in providing patients with a more effective and less invasive treatment option.
The authorization of Casgevy is a testament to the tireless efforts of researchers, scientists, and medical professionals who have dedicated themselves to advancing the field of gene therapy.
Their dedication and perseverance have culminated in the development of a groundbreaking treatment that has the potential to transform the lives of individuals affected by sickle cell disease and thalassemia.
This achievement underscores the importance of continued investment in research and development in the field of gene therapy, as it holds the key to unlocking new and innovative treatments for a wide range of genetic disorders.
As we celebrate this historic milestone, it is important to recognize the far-reaching implications of this achievement.
The approval of Casgevy not only offers hope to individuals with sickle cell disease and thalassemia in the United Kingdom, but it also paves the way for the development of similar treatments for genetic disorders worldwide.
This momentous occasion serves as a reminder of the power of scientific innovation and the potential for gene therapy to make a lasting impact on the lives of countless individuals.
In conclusion, the authorization of Casgevy by Britain’s medicines regulator represents a significant advancement in the treatment of sickle cell disease and thalassemia.
This milestone heralds a new era in the field of gene therapy, offering hope to patients who have long faced limited treatment options.
The approval of Casgevy is a testament to the dedication and perseverance of researchers and medical professionals, and it underscores the potential of gene therapy to revolutionize the treatment of genetic disorders.
As we look to the future, it is essential to continue supporting and investing in research and development in the field of gene therapy, as it holds the key to unlocking new and innovative treatments for a wide range of genetic conditions.
Dr. Helen O’Neill of University College London has stated that the future of life-changing cures lies in CRISPR based gene-editing technology.
This revolutionary technology has the potential to transform the field of medicine by allowing scientists to make precise changes to the DNA of living organisms.
By targeting specific genes, CRISPR has the potential to cure genetic diseases, create new treatments for cancer, and even enhance the human genome. The possibilities are endless, and the potential impact on human health and well-being is immense.
However, it is important to proceed with caution and ethical considerations when using CRISPR technology, as the implications of altering the genetic code of living organisms are far-reaching.
Nevertheless, the potential benefits of CRISPR technology are undeniable, and it is an exciting time for the future of medicine and healthcare.
The recent approval of gene therapy by the MHRA has sparked a significant shift in the treatment of sickle cell disease and thalassemia, both of which have long been considered incurable.
The use of the word ‘cure’ in relation to these genetic disorders has been deemed incompatible in the past. However, with the approval of gene therapy, there is a growing sense of optimism and hope for those affected by these conditions.
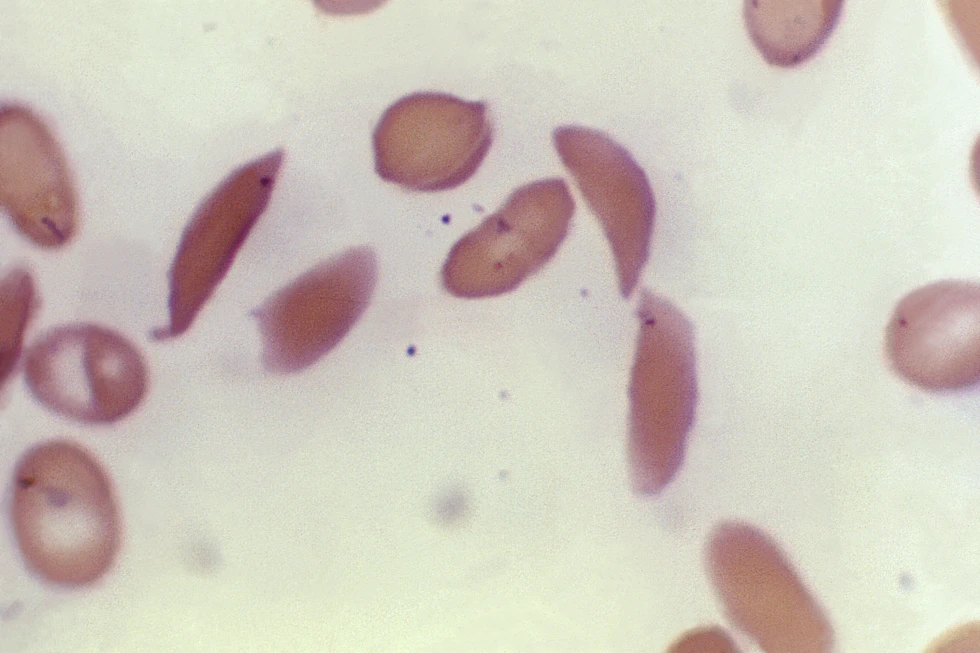
Sickle cell disease and thalassemia are both caused by genetic mutations affecting the hemoglobin-carrying genes, resulting in a range of debilitating symptoms.
Sickle cell disease, prevalent in individuals with African or Caribbean backgrounds, can lead to excruciating pain, organ damage, stroke, and other complications due to the abnormal shape of red blood cells.
On the other hand, thalassemia, which predominantly affects individuals of South Asian, Southeast Asian, and Middle Eastern heritage, can cause severe anemia requiring frequent blood transfusions and lifelong medical interventions.
The approval of gene therapy marks a positive moment in history and offers new hope for those living with these conditions.
The medical community is abuzz with excitement over the potential of a new medicine called Casgevy, which targets the problematic gene in a patient’s bone marrow stem cells, allowing the body to produce properly functioning hemoglobin.
The treatment process involves administering a course of chemotherapy to the patient, followed by the extraction of stem cells from their bone marrow.
These cells are then taken to a laboratory where genetic editing techniques are used to fix the faulty gene before being infused back into the patient’s body for a permanent cure.
Hospitalization is required at least twice, once for the collection of the stem cells and again for the infusion of the altered cells.
Dr. James LaBelle, the director of the pediatric stem cell and cellular therapy program at the University of Chicago, expressed his excitement about the new treatment, stating that it represents a new wave of treatments for patients with sickle cell disease.
He also noted that the recent approval of Casgevy in Britain suggests that authorization in the United States is likely to be imminent.
The U.S. Food and Drug Administration is currently reviewing Casgevy and is expected to make a decision early next month.
Officials at the University of Chicago are already taking steps to build the clinical and reimbursement infrastructure necessary to provide this revolutionary treatment to patients.
The recent decision by Britain’s regulatory body to authorize gene therapy for sickle cell disease and thalassemia has sparked significant interest and debate within the medical and healthcare communities.
This decision was underpinned by a study involving 29 sickle cell disease patients, the vast majority of whom, 28 out of 29, reported a remarkable absence of severe pain issues for a minimum of one year following treatment.
Similarly, in the case of thalassemia, 39 out of 42 patients who underwent gene therapy did not require a red blood cell transfusion for at least a year thereafter.
However, the enthusiasm surrounding these breakthroughs is tempered by concerns over accessibility and affordability. Gene therapy treatments are known to carry exorbitant costs, often reaching into the millions of dollars, thereby raising apprehensions about their accessibility to those most in need.
Notably, the approval of a gene therapy for a fatal genetic disorder in Britain last year, with a list price of £2.8 million ($3.5 million), prompted negotiations by England’s National Health Service to secure a confidential discount, making it accessible to eligible patients.
Vertex Pharmaceuticals, the company behind the therapy, has yet to determine a price for the treatment in Britain and is actively collaborating with health authorities to expedite reimbursement and access for eligible patients.
In the United States, while Vertex has not disclosed a potential price for the therapy, a report by the Institute for Clinical and Economic Review suggested that prices up to around $2 million would be deemed cost-effective.
This stands in stark contrast to research findings earlier this year, which indicated that medical expenses for existing sickle cell treatments, from birth to age 65, accumulate to approximately $1.6 million for women and $1.7 million for men.
The intersection of groundbreaking medical advancements and the financial implications for patients and healthcare systems underscores the complex and multifaceted nature of the ongoing discourse surrounding gene therapy.
The process of approving medicines and treatments in Britain is a rigorous one, involving the scrutiny of a government watchdog before they are deemed suitable for use in the national health care system.
This ensures that only the most effective and safe medications and therapies are made readily available to patients, thereby upholding the highest standards of healthcare.
In the case of sickle cell disease, a condition that affects millions of individuals worldwide, including a significant number in the U.S., the approval process is particularly crucial.
Sickle cell disease is more prevalent in regions where malaria is or was widespread, such as Africa and India, and is also more common among certain ethnic groups, including individuals of African, Middle Eastern, and Indian descent.
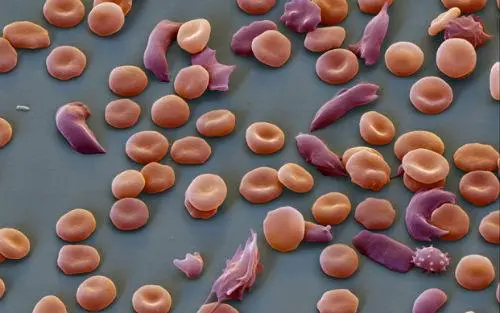
It is believed that carrying the sickle cell trait may provide some protection against severe malaria, highlighting the complex relationship between genetics and disease susceptibility.
Therefore, the thorough evaluation and recommendation of treatments for sickle cell disease are of paramount importance, given its prevalence and the potential implications for public health.
By adhering to stringent regulatory standards, the British healthcare system can continue to provide access to the most effective and safe treatments for all patients, including those affected by sickle cell disease.